- Created by Unknown User (hermanv) on 17.12.2014
You are viewing an old version of this page. View the current version.
Compare with Current View Page History
Version 1 Current »
Let’s first start of with the why, for after that we can establish which is the most energy efficient atom to use for a fusion reaction.
Fusion happens when two nuclei come close enough for the nuclear force to exceed the electrostatic force and pull them together (fuse). This process takes light nuclei and forms a heavier one. For nuclei lighter than iron-56 this is an exothermic reaction and releases energy. Nuclei heavier than iron-56 require an external source of energy for this reaction, making those endothermic reactions. Nuclei smaller than iron-56 are more likely to fuse, whilst heavier nuclei are more likely to break apart (fission). Well known examples of these processes are stars such as our sun, which is powered by fusion, and a nuclear reactor, which uses the energy that comes forward during fission.
To fuse, nuclei must overcome the repulsive Coulomb force, a force caused by the nuclei containing protons that repel via the electromagnetic force. To overcome this barrier, the nuclei must have a high kinetic energy by e.g. heating them up. Once an atom is heated above its ionization energy, its electrons are stripped away leaving the bare nucleus; the ion.
In theory, any atom could be fused, given that enough energy is applied. If an atom has a small charge, less energy is required to fuse such an atom. The less protons an atom has, the smaller the charge is that needs to be overcome for fusion to take place.
The smallest atom that is known to man is hydrogen (and its isotopes), having only one proton - the smallest possible number besides zero. The nuclear binding energy is thus low for hydrogen, which makes it ideal for fusion generated power.
However, the most promising fusion reaction is not with 'regular' hydrogen (1H), but that of deuterium (2H) and tritium (3H). These two isotopes have one, respectively two neurons in the nucleus, besides the proton. As can be seen in the image to the right, performing a fusion reaction between these two isotopes (D-T reaction) results in an excess energy of 17.59 MeV, and the production of 4He. The reaction below, that of 2H+3He, gives an even higher energy result. However, due to the higher Coulomb force of helium the heat required to initiate the reaction is much higher than that of a D-T reaction. Also, the natural abundance of 3He is very limited; 0.000137% of all helium on earth is 3He. Deuterium on the other hand is more abundant (0.0156% of all hydrogen on earth). Natural occurring tritium is very rare, however it is relatively easily produced.
A little something that might be interesting to read and see: http://www.lockheedmartin.com/us/products/compact-fusion.html
Lockheed Martin states that it will have a small nuclear fusion generator ready within five years.
Sources: http://hyperphysics.phy-astr.gsu.edu/
http://en.wikipedia.org/wiki/Nuclear_fusion
www.euronuclear.org/info/encyclopedia/f/fusion.htm


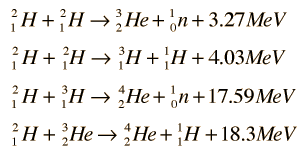

- No labels